On June 27, the Beijing Food and Drug Administration announced the “Detailed Implementation Rules for the Registration of Drugs and Medical Device Products in Beijing (Trial)†(hereinafter referred to as the “Regulationsâ€).
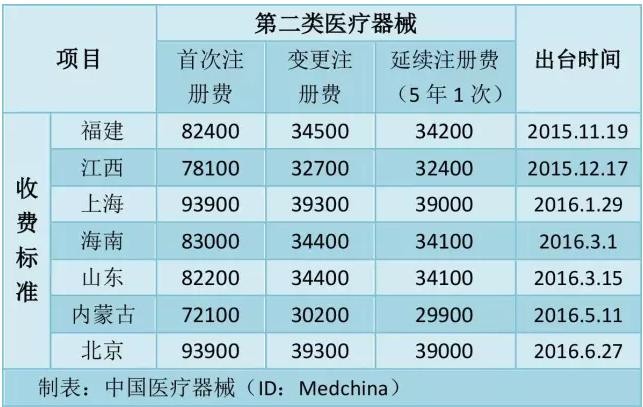
According to the document, the first registration fee for the second type of medical equipment in Beijing is 93,900 yuan, the registration fee is 39,300 yuan, and the registration fee is 39,000 yuan.
Among them, the registration fee for in vitro diagnostic reagents managed by medical devices is also applied to the Regulations.
The relevant charging standards will be implemented as of July 1, 2016. The applicant must pay the registration fee within 5 working days after receiving the "Administrative License Project Payment Notice", otherwise the registration procedure will be terminated by itself.
No charge for 2 cases
If the "Administrative Measures for the Registration of Medical Devices" and the "Administrative Measures for the Registration of In Vitro Diagnostic Reagents" are those for registration change, no change registration fee will be charged.
Small and micro enterprises that meet the requirements of the SMEs Standardization Regulations (Ministry of Industry and Information Technology [2011] No. 300) apply for registration of innovative medical device products, and the first registration fee is waived.
2 cases are not refundable
After the application for registration is accepted, if the applicant initiates the withdrawal of the application for registration, or if the Beijing Drug Administration Department makes a decision not to grant permission, the registered registration fee will not be refunded.
If the application for registration is filed again, the fee shall be repaid.
Beijing and Shanghai are the most embarrassing!
So far, among the seven provinces that have already issued the charging standard, Beijing and Shanghai have the most expensive fees.
Zhejiang Haisheng Medical Device Co., Ltd , https://www.hisernmedical.com