Matthew A. Lauber, Stephan M. Koza, and Kenneth J. Fountain
Purpose <br> Showcases all aspects of the GlycoWorks TM workflow, including the flexibility to apply different SPE patterns in method validation and troubleshooting, and the feasibility of the human IgG control standard used.
Background <br> The glycosylation of proteins is a very meaningful post-translational modification that regulates the structure and function of proteins. Glycosylation of biotherapeutic drugs is undoubtedly a structural feature that must be thoroughly characterized and monitored, especially when changes in the glycan profile are associated with changes in efficacy and/or immunogenicity.
A set of conventional methods for evaluating N-glycans in glycoproteins involves the release of polysaccharides by PNGase F, labeling with fluorescently active 2-aminobenzamide (2-AB), and then hydrophilic interaction chromatography. The method (HILIC) was performed and finally detected by a fluorescence detector (FLR) (Fig. 1). In this workflow, the more complicated one is the sample preparation step. To make this process more intuitive, Waters® introduced GlycoWorks, which combines the many consumables required for N-sugar preparation and analysis. Most notably, GlycoWorks implements the pre/post labeling purification step of HILIC SPE, which is important to ensure the stability of the method. GlycoWorks can help prepare N-glycans for subsequent analysis and provide different SPE types (low/high throughput) and IgG control standards for method validation and troubleshooting.
The following highlights two prominent aspects of the GlycoWorks workflow: for different SPE types (single-column and high-throughput plates), as well as human IgG control standards that can be used for method validation and troubleshooting.
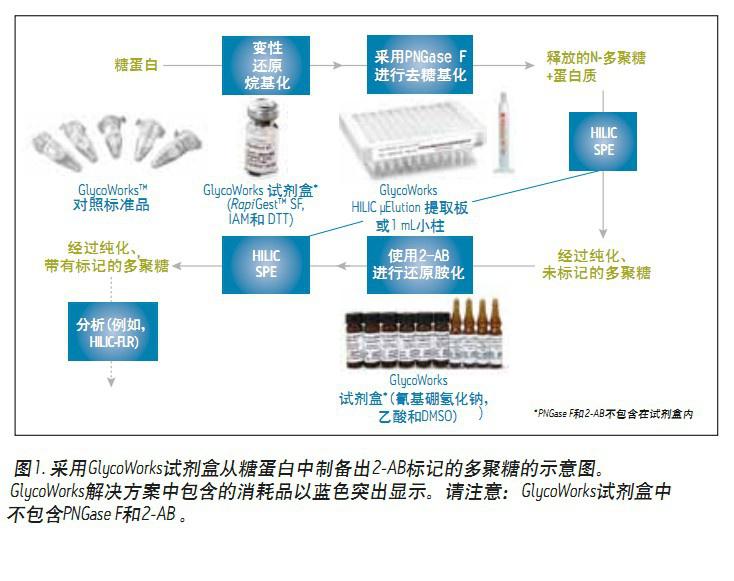
Solution <br> 2-AB labeled N-glycans were prepared from GlycoWorks control standard (p/n 186007033), human IgG, by two different SPE types and each using the protocol provided in the maintenance manual. Figure 2A shows the HILIC-FLR chromatogram of the polysaccharide in the control standard using the GlycoWorks HILIC μElution 96-well plate (p/n 186002780), ACQUITY UPLC GST Amide (BEH Glycan) column, and instrument control and data analysis. UNIFI?. By comparing the glucose unit (GU) value with the polysaccharide performance test standard (part number 186006349) (as with the control standard based on human IgG), the nine most abundant aglycans in this spectrum were performed. distribution.
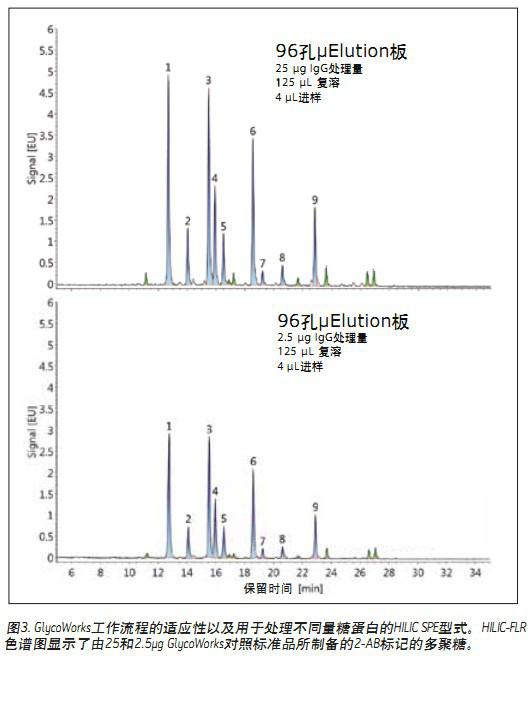
The chromatogram obtained using a GlycoWorks HILIC 1 ml cartridge (single use) (part number 186007080) is shown in Figure 2B. The two chromatograms show very similar results regardless of the SPE type chosen. To verify this result, the assigned peaks were integrated and the relative abundance of the above nine polysaccharides was determined using the peak area (Fig. 2C). This analysis demonstrates that there is no significant difference in the relative concentrations of the polysaccharides obtained using the two different types of SPE. In this study, we also evaluated the effect of sample size on the glycan profile using GlycoWorks control standards. Figure 3 shows, in contrast, a HILIC-FLR chromatogram of a polysaccharide (25 μg to 2.5 μg IgG) prepared using a HILIC μElution plate. The overall recovery of the 2.5 μg sample is below the average and is most likely due to a small amount of non-specific surface loss. Although the amount of sample processed differed by a factor of ten, the relative abundances identified were highly similar, with a difference of < 11%. Among them, peak 9 (G2FS1) had the largest deviation, and when treated with 25 μg and 2.5 μg IgG, the deviation values ​​were 8.5% and 7.6%, respectively.
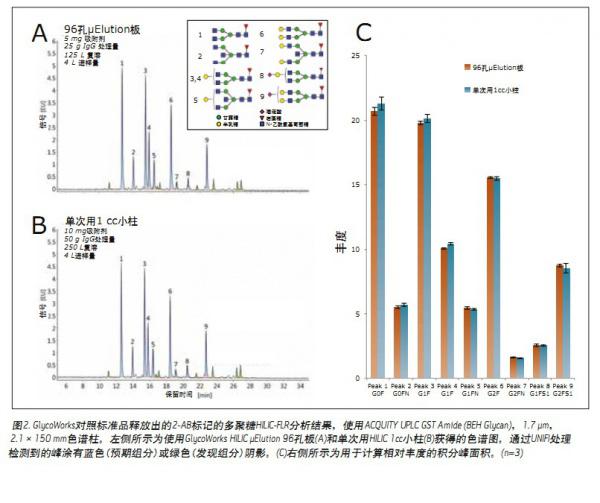
The GlycoWorks workflow is available for two different SPE types: one for high throughput requirements (96-well μElution extraction plates) and the other for low-throughput applications (1 cc cartridges). Most importantly, the results show that no matter what GlycoWorks SPE type is chosen, a very similar glycan profile can be obtained. Experimental Results N-polysaccharides in Glycoworks control standards were analyzed based on the GlycoWorks workflow. This standard is included with the GlycoWorks kit and can be used to confirm the correctness of the results or to potentially assist in the troubleshooting process.
To download a complete and clear application note, please click: http://?locale=122&lid=134741826&cid=511436
Medical Equipment,Irrigation Pump,Stainless Steel Sterilization Cases,Sterilization Basket Box
ZHEJIANG SHENDASIAO MEDICAL INSTRUMENT CO.,LTD. , https://www.shendasiaomed.com