Recently, the Academician Wei Yuquan from the State Key Laboratory of West China Biotherapy of Sichuan University used the artificial virus to carry out the CRISPR-Cas9 gene editing system for the first time. He successfully completed the target gene editing in the mouse tumor model and achieved better tumor treatment. The results, related results were published in the Journal of the American Chemical Society·Nano.
"Nature" magazine about the first time that Professor Lu Yu of the West China Hospital of Sichuan University used CRISPR technology to edit T cells and return it to patients for tumor treatment. I must have seen it. I am amazed at Professor Lu’s courage and foresight. Recently, there is also a new progress in the application of CRISPR application in Huaxi Hospital. The research team of the Academician Wei Yuquan from the State Key Laboratory of Biotherapy in West China used artificial virus for CRISPR-Cas9 delivery for the first time, successfully completing the target gene in the mouse tumor model. The editor has achieved a good tumor treatment effect, and the related results were published in the Journal of the American Chemical Society·Nano.
You may say that human experiments are already underway, and it seems that the mouse experiment is not surprising. Don't forget, the human experiment is to edit the T cells of immune cells in vitro, and then return to the treatment after editing. However, the powerful magic scissors can only be used in vitro to edit T cells. It is a great use of CRISPR knockout tumors in vivo. Genes for cancer treatment are also what researchers are dreaming of. Although there have been many studies on the editing of tumor genes with CRISPR so far, there are many problems in these studies, and the clinical transformation of the methods used is quite difficult. At present, there are mainly three methods for in vivo administration of CRISPR-Cas9 plasmid DNA, one is hydrodynamic injection, that is, DNA plasmid injection of about 10% of mouse body weight is injected through the tail vein in 3-8 seconds. In mice (the condition of the brain to make up the drip), this will cause an increase in blood pressure, which may cause organ damage, which is almost impossible to achieve in the human body. Another way is to use the adenoviral vector for the delivery of CRISPR plasmid DNA. However, adenovirus is immunogenic and has the risk of triggering an immune response. The most important thing is that because the CRISPR plasmid DNA is too large, the adenovirus load capacity is too low, so the efficiency is not high, and the heart is not enough. The third is The shortcomings of local injection of the plasmid are self-evident, and it seems that the deep disease of the human body is beyond the reach.
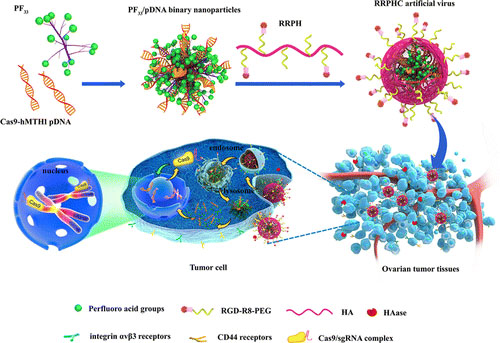
Based on this, Dr. Wei Yuquan has combined into a new type of artificial virus vector for the delivery of CRISPR-Cas9 plasmid DNA. They synthesize the MTH1 gene (a gene that promotes genome stabilization and prevents cell death) in breast cancer. CRISPR-Cas9 plasmid DNA (Cas9-hMTH1), which is highly expressed in breast cancer patients, was experimentally verified in mice. Since the plasmid DNA was negatively charged, they chose a positively charged fluorine-modified polyethyleneimine (PF33) mixed with plasmid DNA to form an artificial virus through positive and negative charge interaction (PF33/ Cas9-hMTH1, which is actually a kind Nanoparticles), and in order to enhance the efficiency of artificial virus delivery, they used a multifunctional polymer RGD-R8-PEG-HA to modify the artificial virus to obtain a multifunctional core-shell structure of CRISPR-Cas9-transporting artificial virus targeting MTH1. (RRPHC/Cas9-hMTH1), this polymer can make the artificial virus more stable, while avoiding the elimination by the immune system. RGD and HA can simultaneously target the integrin ανβ3 and CD44 receptors that are highly expressed in the tumor site, thereby improving labor. Enrichment of the virus at the tumor site. At the same time, the transparent acid (HA) enzyme with high expression in the tumor site can degrade HA and promote the release of Cas9-hMTH1 in the tumor, thereby enhancing the therapeutic effect.
Explosion-Proof System,Combustible Gas Detector,Fire Gas Alarm Detector,Ultraviolet Flame Detector
LIAONING YINGKOU TIANCHENG FIRE PROTECTION EQUIPMENT CO.,LTD , https://www.tcfiretech.com