Tail tip blood collection
- After anesthetizing the mice, wipe the tail with warm water to cause a slight vasodilation. But the water temperature should not be too high.
- Use a sterile scalpel, a blade or sharp scissors to quickly cut off the tip of the mouse by 0.5-1 cm. If you need to collect blood several times, you only need to cut off 2-3mm each time.
- You can massage from the tail to the tip of the tail to increase blood flow. However, this will reduce the quality of the blood sample and increase the risk of hemolysis.
- Blood can be collected from the capillaries or directly into the collection tube.
- After the blood collection is over, press the wound or use a hemostatic agent (such as silver nitrate) to stop the bleeding.
- The amount of blood collected each time is up to 0.1ml.
Eyelid venous plexus
- After gas anesthesia in mice, the left thumb and forefinger oppressed the sides of the neck of the mouse, causing the eyeball to protrude and the venous plexus of the eyelid to be congested. The mouse can also be placed in the lateral position, with the thumb and index finger placed on the top of the mouse and the lower jaw, respectively, and the skin pulled back and down.
- Avoid stressing the trachea when grasping, otherwise it may affect the breathing of the mouse.
- The capillary blood collection tube is placed at the inner corner of the eye and pierced at a depth of 30-45 degrees with the plane of the nose.
- Apply pressure while gently rotating the blood collection tube. The blood will flow into the blood collection tube by capillary action.
- Can not be punctured too deep, usually 2-3mm.
- Immediately after the blood collection, immediately release the pressure on the mouse to reset the eyeball and pull out the blood collection device.
- Use a dry cotton ball to hold the eyelids to ensure hemostasis.
Generally, the blood volume of the orbital venous plexus can reach 0.2-0.3ml.
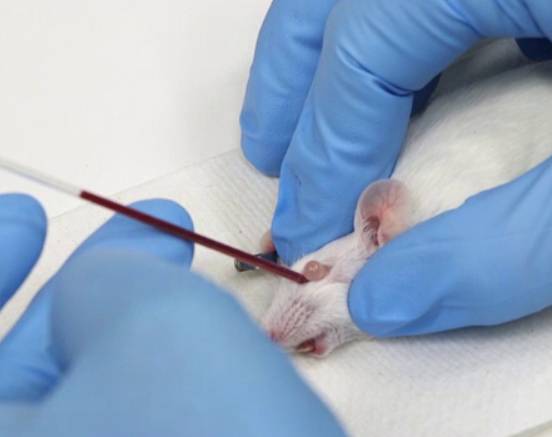
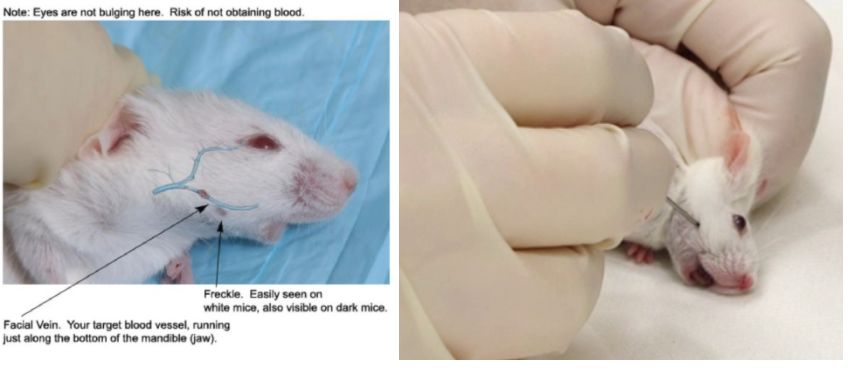
Submandibular vein blood collection
- In Baoding mice, the mice can be placed on their side to make the mouse head as fixed as possible.
- The approximate location of the submandibular vein is found along the rubber at the corners of the mouse's mouth and the outer corner of the eye. A small hairless spot is found, a bit like a dimple. Basically located at the distal end of the mouth slightly below the chin line.
- The needle remains perpendicular to the surface of the skin and penetrates the skin. The depth does not exceed the slope of the needle.
- After the needle is pulled out, the blood will flow out. To facilitate blood collection, the head of the mouse can be lowered below the heart height.
- The blood can be dripped directly into the collection tube, or a capillary blood collection tube can be used.
- After the blood collection is finished, press to stop bleeding.
Generally, the blood collection volume is 0.2-0.5 ml.
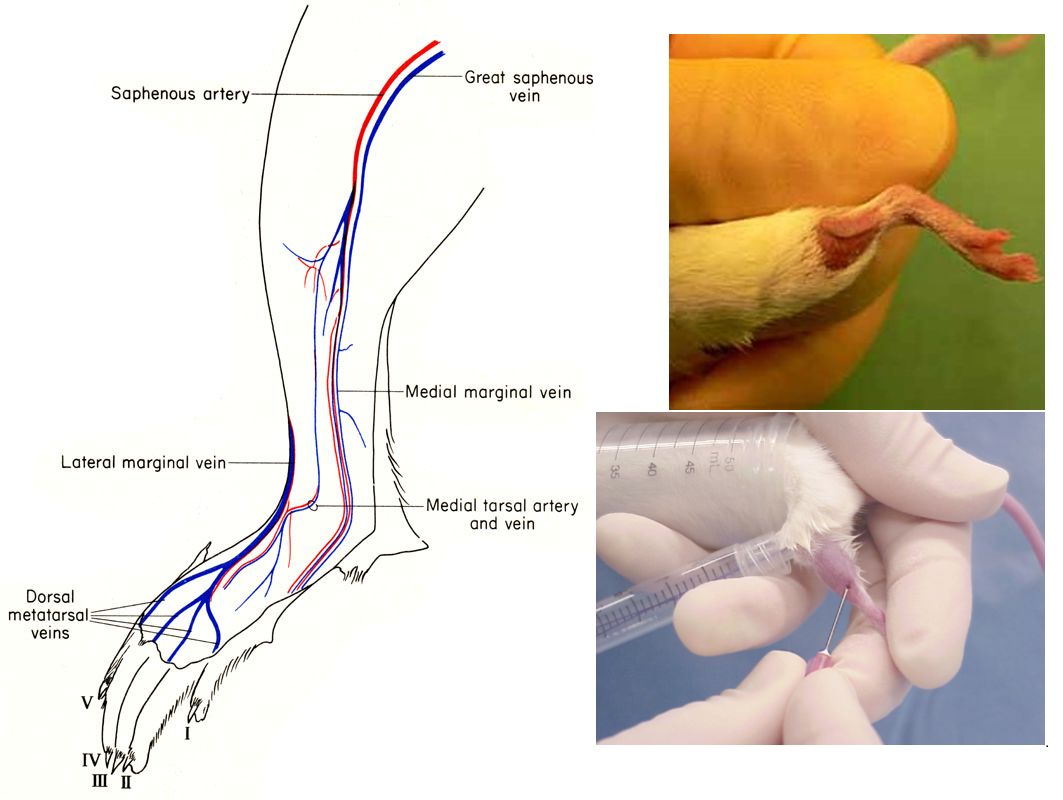
Saphenous vein blood collection
- Place the mouse in a fixed tube or rack, but keep the hind legs free to move and shave the hair of the ankle.
- To make the saphenous vein between the knee and ankle more visible, a tourniquet can be used above the knees of the hind legs. The saphenous vein can be found on the skin surface near the tail.
- The needle pierces the blood vessel perpendicular to the surface of the skin. Do not put the needle too deep to avoid piercing the muscles or touching the bones.
- The blood will slowly flow out from the needle, collect it with capillary blood collection tubes, and loosen the tourniquet.
After the blood collection is completed, the wound is pressed or a hemostatic agent (such as silver nitrate) is used to stop the bleeding, and the mouse is returned to the cage.
Cardiac blood collection
- There are generally three types of heart blood collection postures:
1. Lift the torso of the mouse so that the body is perpendicular to the ground. The body is upright to prevent deflection of the heart or distortion of the chest. Use a 1 ml syringe and a 22g needle. Insert the needle 5 mm down the center of the chest, 5-10 mm deep, and hold the syringe and chest at 25-30 degrees.
2. The mouse is placed supine and the needle is inserted vertically through the sternum.
3. Place the mouse on its side and insert the needle perpendicular to the chest wall. - If the blood does not enter the syringe immediately, gently pump the needle to create a vacuum zone.
- When blood is present in the syringe, hold the needle still and gently pull the plunger to obtain more blood.
Generally, 0.8-1.0 ml of blood can be taken.

references
1. Diehl K. H, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 21, 15-23. (2001).
2. Joslin OT, et al. Blood collection techniques in exotic small animals. Journal of Exotic Pet Medicine. 18:2. 117-139.(2009)
3. Janet H, et al. Methods of Blood Collection in the Mouse. Lab Animal. 29 (10): 47-53. (2010).
4. Guidelines for the Effective bleeding of mice and rats. (2000).
5. Hem A., et al. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Laboratory Animals. 32. 364-368. (1998)
Waist massager
Waist massager
Shenzhen Jie Zhong Lian Investment Co., Ltd. , https://www.szmeizons.com